The latest
Keep up with our news, insights, project updates, and commentary.
Catalyst Global To Conclude Transformative Decade of Increasing Access to Sexual and Reproductive Health Products and Services for Girls and Women Globally
After more than a decade of dedication to increasing access to critical sexual and reproductive health products and services in the global South, Catalyst Global (formerly WCG Cares), announces its decision to cease operations in 2024.
ICYMI: Here’s what people are saying about the Contraceptive Innovation Index
Contraceptive product introduction is complex. The Contraceptive Innovation Index, designed to facilitate discussions and decision-making around the introduction and scale up of contraceptive technologies, offers a comprehensive but streamlined way to organize and make sense of information.
Catalyst Global Announces New Board Member Dr. Jotham Musinguzi
The non-profit organization welcomes an award-winning expert in population and development, reproductive health, family planning and HIV/AIDS.
Overwhelmed with health product registration? We’re here to help!
Regulatory requirements for product registration can be overwhelming. They’re complex, vary by country, and frequently change. We know they’re important (safe medicines, yes!), but what does it actually take to get a product from the manufacturing plant onto the shelves in your local pharmacy? Let’s take a look together.
Introducing Catalyst Global
We have a new name and are rebranding WCG Cares as Catalyst Global.
EECO Product Registration Toolkit
The Product Registration Toolkit is a digital collection of adaptable resources to guide the process of registering health products, like contraceptives, in low- and middle-income countries.
Market Access Accelerator
The goal of the Market Access Accelerator is to support commercial partners to bring new, innovative sexual and reproductive health products to market in low- and middle-income countries.
Comprehensive Regulatory Support
Nearly 2 billion people in the world lack access to essential medicines (WHO, 2019). In many low- and middle-income countries, safe and effective medicines are not always available, accessible, or affordable, which can lead to preventable injury and death.
Quality Assurance (QA) Support
Quality Assurance (QA) of drugs and medical devices is a major public health challenge. People need medicines that are manufactured in compliance with the global regulations and safety standards; however, substandard and/or counterfeit pharmaceutical products and medical devices are widely accessible in many countries around the world.
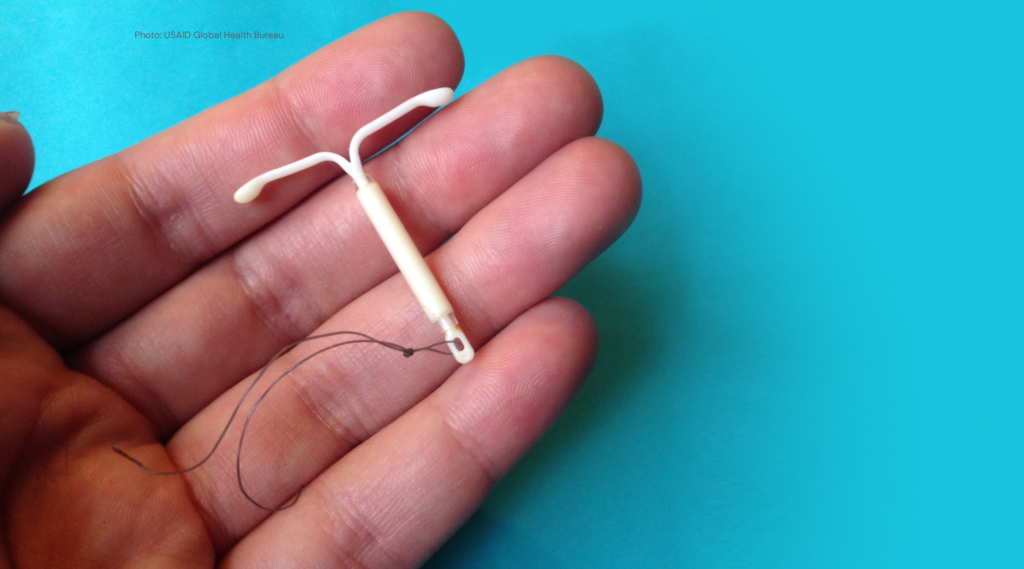
Learning about Expanded Access and Potential of the Levonorgestrel Intrauterine System (LEAP LNG-IUS)
With funding from the Bill & Melinda Gates Foundation, FHI 360 and partners, Catalyst Global and Population Services International (PSI) are implementing the Learning about Expanded Access and Potential of the LNG-IUS (LEAP LNG-IUS) Initiative.
The Tryst Network Campaign
The Tryst Network provided a sex-positive pool of resources to improve the sexual health IQ of women and their partners through fun, reliable and empowering online content.
Maximizing Health Care Provider Performance (MAX)
The MAX intervention worked with health care providers to address individual and structural barriers constraining sexual and reproductive health services and contraception provision in Kenya and South Africa.
Dual Prevention Pill (DPP) Acceptability Study
With funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the Expanding Effective Contraceptive Options (EECO) project, Catalyst Global collaborates with Population Council and the Wits Reproductive Health and HIV Institute on the implementation of an acceptability study of the Dual Prevention Pill (DPP), a multipurpose prevention technology that combines oral pre-exposure prophylaxis (PrEP) and an oral contraceptive, in South Africa.