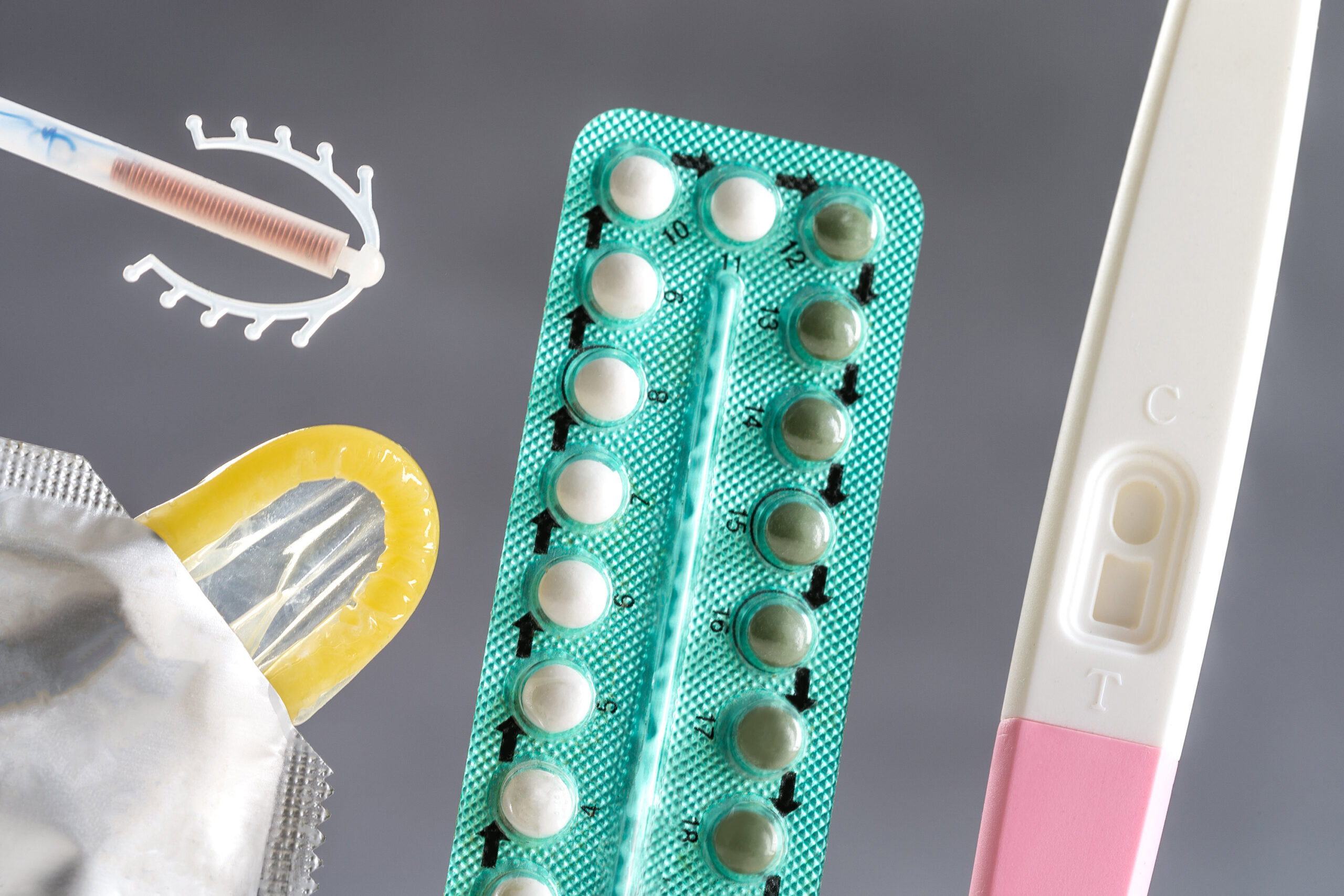
The goal of the Market Access Accelerator is to support commercial partners to bring new, innovative sexual and reproductive health products to market in low- and middle-income countries.
Quality Assurance (QA) Support
Quality Assurance (QA) Support
Such products are at best ineffective, resulting in the growth of drug resistance and prolonged or ineffective treatment for patients, and at worst they are dangerous, putting lives at risk.
Our Quality Assurance Team collaborates with manufacturers, suppliers and partners to ensure the delivery of safe, effective sexual and reproductive health products, and maintain compliance to global regulatory requirements.
What we do
- Collaborate with manufacturers, suppliers and partners to ensure the delivery of safe, effective products, and ensure compliance to global regulatory requirements.
- Provide technical assistance to manufacturers, including help with technology transfers and the development of standard operating procedures, to help improve their quality management systems.
- Audit each supplier prior to commercial distribution to assure compliance with the applicable country regulations.
- Conduct cGMP assessments to determine the manufacturer’s state of compliance, which includes verification of current regulatory status and performance of an onsite cGMP audit at the manufacturing facility.
- Verify inspection and testing or compliance to product specification for each production lot, and issue corrective action requests to manufacturers to address any identified nonconformities.
- Coordinate and evaluate quality control data for product release.
- Monitor distributed products for post-market surveillance and assist manufacturers with any adverse report issue.
Countries:
Worldwide
Products:
Gynocular colposcope, MVA, Signos RT, Softcup, Caya® Diaphragm and Caya® Gel, AVIBELA, Progesterone Vaginal Ring, Woman’s Condom
“WCG Cares (now Catalyst Global) EECO project has worked with Medintim to expand access to Caya® Diaphragm in LMICs. Their team successfully managed country registrations, procurement, QA monitoring and built evidence for this new nonhormonal product through pilot introductions in Niger and Benin. We commend WCG Cares for their regulatory and product introduction expertise, their strategic partnerships, and applaud their commitment to expanding access to new contraceptive methods.”
Martin Kessel, CEO RA Director
Medintim
Related posts
Expanding Effective Contraceptive Options (EECO)
The EECO project supports the introduction of new and underused contraceptive methods to address method-related reasons for non-use and better meet the reproductive health needs of girls and women.