Contraceptive product introduction is complex. The Contraceptive Innovation Index, designed to facilitate discussions and decision-making around the introduction and scale up of contraceptive technologies, offers a comprehensive but streamlined way to organize and make sense of information.
Learning about Expanded Access and Potential of the Levonorgestrel Intrauterine System (LEAP LNG-IUS)
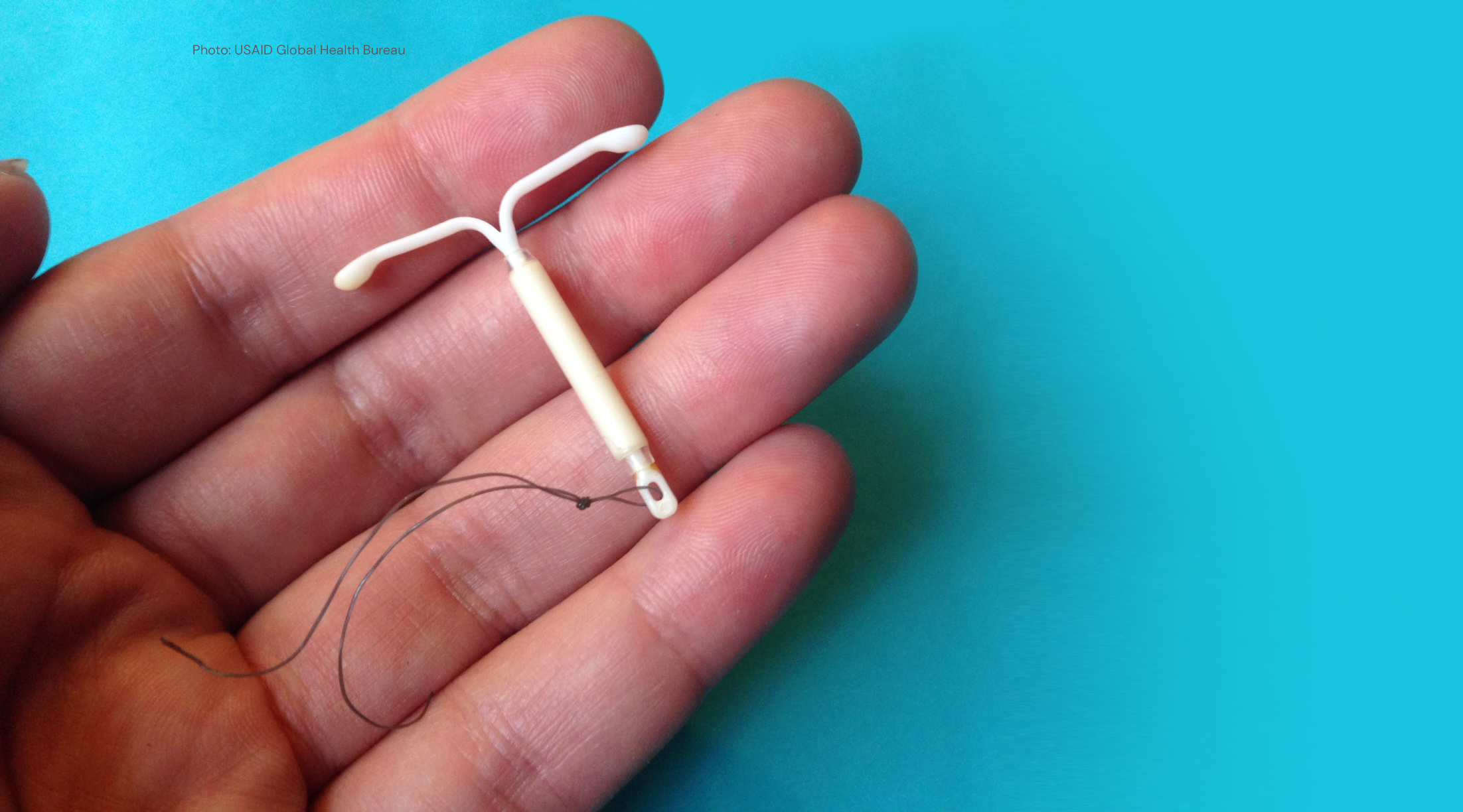
Learning about Expanded Access and Potential of the Levonorgestrel Intrauterine System (LEAP LNG-IUS)
The project is intended to help determine if and how expanded access to the levonorgestrel intrauterine system (LNG-IUS) — a long-acting contraceptive — could increase contraceptive use and continuation rates in sub-Saharan Africa. The evidence generated through the LEAP LNG-IUS initiative will help improve decision-makers’ understanding of the potential value of adding the LNG-IUS to the contraceptive method mix in sub-Saharan Africa and could help to inform national introduction and scale-up plans.
Under the LEAP LNG-IUS Initiative, Catalyst Global conducted a regulatory assessment with the goal of identifying potential strategies to expedite national registrations of LNG-IUS product(s) in Family Planning 2020 (FP2020) countries. Catalyst Global evaluated the potential benefit of pursuing LNG-IUS registrations via national-level regulatory authorities; through regional harmonization initiatives; and by following the World Health Organization (WHO) collaborative procedure, assessed three countries (Ghana, Ethiopia and Vietnam) as case studies.
Catalyst Global is also leveraging the registration of Medicine360’s hormonal IUS, AVIBELA, in Tanzania and Uganda* through the East African Community – Medicines Regulatory Harmonization (EAC-MRH) program in order to create access to AVIBELA in two additional countries (Uganda and Rwanda). The hormonal IUS is the first contraceptive product to be evaluated by the EAC-MRH program, and Catalyst Global will develop a case study based on this experience to share with the reproductive health community.
*Registration in Tanzania and Uganda is supported by the USAID-funded EECO project. Development of the case study is co-funded by the EECO project.
Product:
LNG-IUS
Countries:
Uganda, Rwanda
Project duration:
2018 – 2022
Funders:
Bill and Melinda Gates Foundation
“WCG Cares (now Catalyst Global) is one of our favorite partners. For our drug/device combination product, they streamline and simplify complex regulatory submissions in low-and-middle-income countries. Their expert support is delivered with transparency, collaboration, reliability, and creative problem-solving; all of which greatly increases the efficiency of our small team.”
Sally Stephens, Chief Business Officer, Medicines360
and President, Impact RH360
LEAP Project Reports
- Learning about Expanded Access and Potential of the Levonorgestrel Intrauterine System (LEAP LNG-IUS) REGULATORY ASSESSMENT (Nov 2018). https://marketbookshelf.com/wp-content/uploads/2019/05/LEAP-LNG-IUS-Report_WCG_FHI360_Final_Nov2018-v.topost.pdf
Related posts
Overwhelmed with health product registration? We’re here to help!
Regulatory requirements for product registration can be overwhelming. They’re complex, vary by country, and frequently change. We know they’re important (safe medicines, yes!), but what does it actually take to get a product from the manufacturing plant onto the shelves in your local pharmacy? Let’s take a look together.
EECO Product Registration Toolkit
The Product Registration Toolkit is a digital collection of adaptable resources to guide the process of registering health products, like contraceptives, in low- and middle-income countries.