Introducing a product in a new market brings a number of challenges. These can include understanding market opportunities; developing a cost-effective regulatory strategy that is compliant with individual country guidelines; ensuring a steady supply of high-quality products; garnering support from key stakeholders who can help to facilitate the integration of the product; making connections with potential distributors; and understanding the needs and preferences of the girls and women who may use the product. We can help your team to simplify the introduction process and open doors.
Market Access Accelerator Process
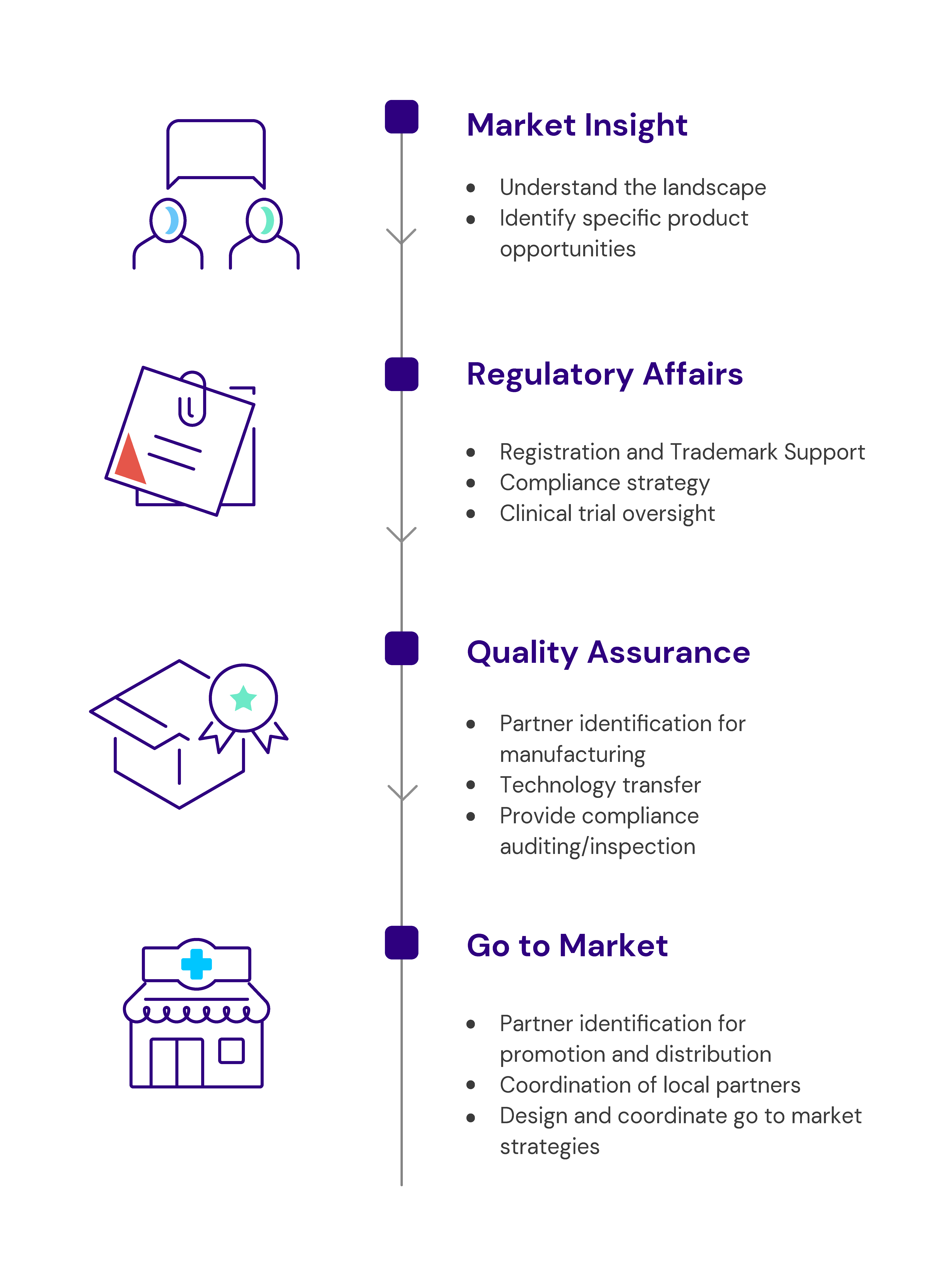
Catalyst Global offers the following services, as a whole or in part, as needed to help you meet your goals.
Market Insights
We map the landscape for critical information:
- The market structure, channels and key players
- Requirements to register a product for use in commercial, public, and other channels
- Private and universal health insurance coverage
- Existing initiatives to create awareness of relevant health area
We conduct assessments to find key insights:
- Wants, needs and perspectives of girls and women
- Gaps that the existing market is not addressing
- Specific products with potential for product-market fit
Regulatory Affairs
- Recommend regulatory compliance strategy
- Compile, review and publish regulatory dossiers for local market authorizations and through regional harmonization mechanisms
- Support products undergoing the WHO prequalification process
- Liaise with National Regulatory Agencies and key local stakeholders to garner support for new product registration and introduction
- Provide scientific advice and clinical trial oversight to products in this stage of development
- Support regulatory advertising and promotion
- Support filing for trademark
- Support for product (re-)classification:
– Integration into Ministry of Health protocols, e.g. list of essential drugs
– Over-the-counter status or new, approved uses
Quality Assurance
- Provide compliance auditing/inspection (internal and external)
- Support the development of quality management systems
- Assist manufacturers to improve quality assurance and quality control
- Oversee quality control testing and batch release
- Support stability programs
- Support technology transfer
Go to Market
- Design, convene and coordinate go-to-market strategies
- Identify the best-suited distribution and promotion partners for each channel
- Provide technical support to promote products to health workers and girls and women
- Assess the potential for and advocate to integrate products into national health care systems
For more information, please contact Shannon Bledsoe: [email protected]




